Abstract
Introduction: Concizumab is currently in clinical development as a humanized, monoclonal anti-tissue factor pathway inhibitor antibody that enhances the initiation phase of coagulation. In the phase 3 explorer7 study (NCT04083781), patients with hemophilia A or B with inhibitors received once-daily subcutaneous prophylactic treatment with concizumab for the prevention of bleeding episodes. Patients with hemophilia generally report impaired health-related quality of life (HRQoL), particularly in the areas of pain and physical functioning. Here, we present an analysis of changes in reported HRQoL for patients treated with concizumab for 24 weeks in the explorer7 study.
Methods: A total of 133 male patients aged ≥12 years were randomized to no prophylaxis (arm 1) or concizumab prophylaxis (arm 2) or assigned to non-randomized concizumab prophylaxis (arms 3 or 4) depending on the treatment they received before entering the study. All patients were asked to complete the 36-item Short Form Health Survey (SF-36v2; Ware & Sherbourne. Med Care 1992; 30:473-83) and patients aged ≥17 years could complete the Hemophilia Quality of Life Questionnaire for Adults (Haem-A-QoL; von Mackensen & Gringeri, Handbook of Disease Burdens and Quality of Life 2010:1896-920; von Mackensen et al.Haemophilia 2017;23(3):383-391; Wyrwich et al.Haemophilia 2015;(5):578-84). Both questionnaires could be completed at baseline (Week 0), and at Weeks 4, 8, 16 and 24. The SF-36v2 health scales "bodily pain" (BP) and "physical functioning" (PF) were analyzed as key secondary endpoints. Changes in Haem-A-QoL total score and "physical health" score, as well as the remaining SF-36v2 health scales and component summary scores, were analyzed as exploratory endpoints. Unfortunately, due to technical issues, several patient-reported outcomes recordings could not be collected for statistical analysis. As the preplanned statistical analysis could not be implemented, a post-hoc mixed model for repeated measures (MMRM) was applied after the randomization code was broken. The MMRM analysis included patients from arms 1 and 2 who had completed the questionnaire at baseline and had at least one post-baseline visit on the new dosing regimen.
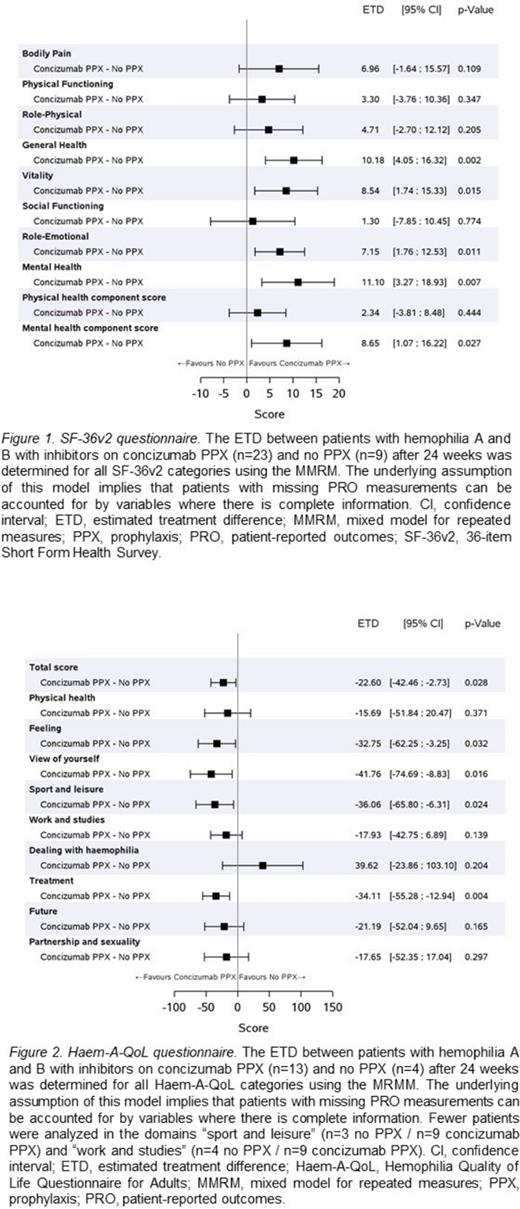
Results: The mean estimated change in score from baseline to Week 24 on the SF-36v2 BP scale was 2.2 points (95% CI: -5.1; 9.5) for patients not on prophylaxis (n=9), and 9.2 points (95% CI: 5.1; 13.3) for those on concizumab (n=23), which yielded an estimated treatment difference (ETD) of 7.0 points (95% CI: -1.6; 15.6) (Figure 1). For the SF-36v2 PF scale, the mean change in score from baseline to Week 24 was 1.2 points (95% CI: -4.8; 7.1) in the no prophylaxis arm (n=9) versus 4.5 points (95% CI: 0.8; 8.2) in the concizumab treatment arm (n=23), which yielded an ETD of 3.3 points (95% CI: -3.8; 10.4) (Figure 1). While BP and PF scores were not significantly different between both arms, several other health scales, including "general health", "mental health", "role-emotional" and "vitality", showed statistically significant differences (Figure 1). The total score from the Haem-A-QoL questionnaire showed a significant improvement for patients on concizumab prophylaxis (n=13) compared to no prophylaxis (n=4) (ETD: -22.6 points [95% CI: -42.5; -2.7]; Figure 2). A significant difference was not observed for "physical health", but there were significant improvements in "feeling", "treatment", "view of yourself", and "sport and leisure" (Figure 2).
Conclusion: Patients on daily subcutaneous concizumab prophylaxis generally reported better scores on generic (SF-36v2) and disease-specific (Haem-A-QoL) questionnaires when compared with patients treated on-demand. The differences were mostly in favor of concizumab, with the largest ETDs being related to mental and general health. SF-36v2 and Haem-A-QoL are being investigated as part of the explorer8 concizumab phase 3 study in patients with hemophilia A or B without inhibitors. These data reflect the potential benefit of concizumab prophylaxis to improve HRQoL in patients with hemophilia A and B with inhibitors.
Disclosures
Shapiro:Sigilon: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ/Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sangamo Biosciences: Consultancy; Pfizer: Honoraria, Research Funding; Takeda: Research Funding; Freeline: Research Funding; Novo Nordisk Hemophilia Foundation: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kedrion Biopharma: Research Funding; Indiana Hemophilia and Thrombosis Center, Inc: Current Employment. Linari:Roche: Speakers Bureau; Sobi: Speakers Bureau; CSL-Behring: Speakers Bureau; Takeda: Speakers Bureau. Neergaard:Novo Nordisk: Current Employment. Odgaard-Jensen:Novo Nordisk: Current Employment. Thaung Zaw:Novo Nordisk: Current Employment. Tran:Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.